Reinvestment: working on four major race tracks, with a R&D input far beyond the industry average
Over the past years, Haohai Biological Technology has been increasing its investment in ophthalmic business and relaying upon technological innovation to create future growth engines of its ophthalmic business. In 2017-2019, the company’s R&D input in ophthalmic business grew remarkably, to 20.4862 million Yuan, 29.2229 million Yuan and 48.1804 million Yuan respectively, far beyond the comparable listed companies in the industry, with a CAGR of 53.36%.
Currently, Haohai Biological Technology has over 10 ongoing R&D projects in the four therapeutic fields of cataract, optometry, ocular surface and ocular fundus, covering such blockbuster products as intraocular lens, orthokeratology lens, new-type artificialvitreous body and Moxifloxacin HCl Ophthalmic Solution.
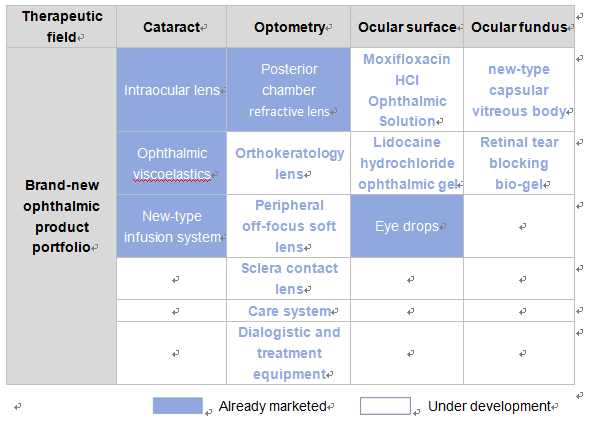
Of them, many ongoing R&D projects including orthokeratology lens and hydrophobic mold injection aspheric intraocular lens have entered the phase of clinical trial or filing for production.
Cataract: aiming at developing high-end intraocular lens to accelerate the import substitution
Cataract is the world’s number one blinding eye disease, and surgically implanting intraocular lens is the only way to treat it at present. According to the “Word Report on Vision” published by the IAPB in 2019, it is estimated that China’s cataract patient population (aged 45-89) will reach 132 million by 2020. This also means there is a huge market demand for intraocular lens in China.
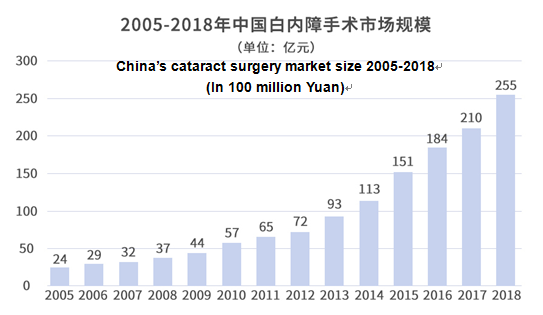
Calculated based on the number of cataract surgeries and per capita medical expenses of cataract surgery at public hospitals in 2005-2018 as published by the “Looking at the progress of cataract prevention and treatment in China from big data”, thanks to the fast growth of cataract surgeries and steadily increased per customer transaction, China’s cataract surgery market has grown from 2.4 billion Yuan in 2005 to 25.5 billion Yuan in 2018, a more than tenfold growth.
However, intraocular lens markets inChina, especially the high-end ones, have long been monopolized by imported brands, causing heavy economic burdens to patients with formidably high prices of imported products. To break the market landscape monopolized by imported brands and realize import substitution of intraocular lens, Haohai Biological Technology has established a business portfolio across the entire industry chain from raw material preparation, optical design, innovative technology development to mass production and marketing with respect to intraocular lens since 2015.
Currently, the intraocular lens products marketed and represented by Haohai Biological Technology have formed good product positioning complementation among brand, technology and price differentiations. While relying upon its innovative R&D network featuring interaction at home and abroad, the company’s ongoing ophthalmic R&D projects still covers multilevel products, expected to further meet more clinical application needs in the future.
Myopia prevention and control and refractive surgery: internally-developed orthokeratology lens have entered the clinical trial phase
In addition to the considerable growth potential of the intraocular lens market, the myopia prevention and control and refractive surgery that Haohai Biological Technology is working on also have a tremendous market potential.
According to authoritative statistics, there are nearly 700 million myopic individuals in China, making China the largest myopic country and also the country with the highest myopia incidence, with a 67% myopia incidence among urban teenagers. In addition to wearing glasses (frame glasses or contact lens), correcting myopia using orthokeratology lens has been increasingly recognized by myopic population in China.
According to the statistics released by the ophthalmology and optometry branch of China Association for Medical Devices Industry, 643,000 pairs of orthokeratology lens were sold in China in 2015, with a CAGR of about 44.11% from 2011 to 2015. Based on calculation, however, about 1.08 million people wore orthokeratology lens in 2019, registering a penetration rate of 1.05%, which is still relatively low.
Currently, Haohai Biological Technology is actively developing such ophthalmic products as orthokeratology lens, sclera contact lens, peripheral off-focus soft corneal contact lens and aqueous humor permeable posterior chamber phakic refractive lens using the internally-developed lens design system and based on the highly permeable materials and silicone hydrogel material developed by Contamac.
Of them, the orthokeratology lens product developed by the company is in the process of clinical trial. Meanwhile, the company is working on upgrading China’s only domesticposterior chamber phakic refractive lens in China that is used to correct myopia, with the second-generation aqueous humor permeable posterior chamber phakic refractive lens about to enter the registration and validation process, the vision correction range of which will be more extensive than the previous generation.
Ocular surface: expected to be marketed soon! Moxifloxacin HCl Ophthalmic Solution is under technical review and evaluation
While working intensively on research and development of ophthalmic medical devices such as intraocular lens and orthokeratology lens, Haohai Biological Technology is actively competing for the ophthalmic medication market.
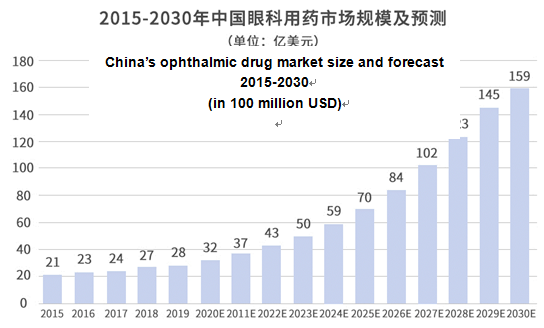
According to a Frost & Sullivan research report, China’s ophthalmic drug market has been growing rapidly, from 2.1 billion USD in 2015 to 2.8 billion USD in 2019, with a CAGR of 8.0%. It is expected that this figure will further grow to 5.9 billion USD in 2024 with a CAGR of 16.0% from 2019 onwards, and further to 16.9 billion USD in 2030 with a CAGR of 19.1% from 2024 onwards.
Similar to the ophthalmic medical device market, the domestic ophthalmic mediation market is also dominated by imported brands, although the market size is huge.
Take the Moxifloxacin HCl Ophthalmic Solution that Haohai Biological Technology is filing for production as an example, this drug is the fourth-generation fluoroquinolones drug and one of the prevalent medicines used to treat bacterial conjunctivitis. Compared with its previous generations, the fourth-generation fluoroquinolones drug has many advantages such as a broader active spectrum, longer duration of activity and smaller possibility of triggering antibiotics resistance.
According to the Frost & Sullivan research, the bacterial conjunctivitis incidence in China was about 29.4% in 2019, with related therapeutic drugs enjoying huge market demands. However, in the domestic market, only one foreign player Novartis is licensed to produce and sell Moxifloxacin HCl Ophthalmic Solution, while other drugs that can be used to treat bacterial conjunctivitis are also manufactured mainly by foreign players such as Santen, Bausch & Lomb and Allergan. I
Moreover, the company has completed the clinical study of lidocaine hydrochloride ophthalmic gel used for local anesthesia in ophthalmic surgeries, a product that can increase the maintenance time of eye-specific surface anesthetic.
Ocular fundus: a revolutionary therapeutic product for rhegmatogenous retinal detachment under development is the first of its kind at home
As a company committed to creating a world-class multinational ophthalmic business group, Haohai Biological Technology has related products marketed in three major therapeutic fields of cataract, photometry and ocular surface. Meanwhile, with an eye on the ocular fundus disease treatment market that started rather late at home, the company entered the market by developing new-type artificial vitreous body and retinal tear blocking bio-gel, thus further optimizing its presence along the ophthalmic industry chain, expanding its competitive edge of ophthalmic product line and injecting new vitality into sustainable development of its ophthalmic business.
Among them, the retinal tear blocking bio-gel under development is the first of its kind in China, capable to cover and adhere to retinal tears and mainly suitable for treatment of rhegmatogenous retinal detachment. Characterized by acute onset and rapid development, this disease, once occurring, will cause vision of patients to rapidly decline and its blinding rate is almost 100% if not treated in time and effectively. According to the “Preferred Practice Pattern (PPP,) 3rd edition, 2018”, the incidence of rhegmatogenous retinal detachment is 10-18 persons/100,000 population, meaning that about 140,000 to 250,000 people contract this disease in China each year.
Compared with the traditional treatment that requires patients to keep a prone position for 1-3 months after surgery in order for fillings to support and press retina to the maximum extent, the retinal tear blocking bio-gel, as a revolutionary treatment method, avoids the suffering of patients required to lie prostrate for a long time after surgery and can extremely facilitate the recovery and normal life of patients. In addition, this product has numerous advantages such as lowered incidence of post-surgery cataract and proliferative vitroretinopathy, avoidance of second surgery (when compared with the traditional silicone implanting) and increased success rate of surgical replacement.
Moreover, the new-type aritificial vitreous bodyof the company is also at the preclinical study phase. The “artificialvitreous body”, known as the blessing for retinal disease patients, can almost be applied in every retinal surgery. Currently, in the domestic markets, most vitreous cavity filling products temporarily support pathological retina mainly through introducing “foreign objects”, while the capsular vitreous body products being developed by Haohai Biological Technology are dedicated to simulating the normal physiological structure of human eyes to the maximum extent, thus achieving the best possible effects in terms of penetration of light ray and fitting of retina and then improving the surgical effects and helping patients realize the best after-surgery vision.

News Center
All Rights Reserved | Haohai Biological Technology | People's Republic of China Internet Medical Products Information Qualification Certificate | Certificate No: (Hu) – Non-Operative-2018-0079
Copyright © 2020-2024 All Rights Reserved Website by Globalquincy.com